- Viewed - 2019
- Printed - 0
- Emailed - 3
- PDF Downloaded - 215
Amniotic membrane - A Pathbreaking Membrane
Year : 2021 | Volume : 1 | Issue : 0 | Page :
MJWI.2022/94
Dr. Ranjana A. Pande , Dr. Padmapriya V ,
Date of Web Publication 31-Mar-2022
Keywords
Amniotic membrane, biological graft, ocular surgery, reconstruction
Introduction:
The eye is a highly sensitive and exposed mucous membrane. The maintenance of the tear film and the transparency is essential to perform the main function-vision.1 Any internal pathology or external insult that breaks this balance makes the eye unable to see and causes extreme disturbance to the patient. The prevalence of ocular surface pathologies is as high as 32%.2 Keeping in mind this large number, it is essential that there be some adequate treatment available to alleviate the symptoms and restore comfort and vision to these patients.
There have been multiple efforts in the past to use membranes, both natural and synthetic to replace the mucous membrane of the eye. Foremost among these were the trial of oral, labial and vaginal mucous membranes. Peritoneum of rabbits and other animals were also tried. The concept of using amniotic membrane was first brought in by Davis for skin transplantation in 1910.3 Ophthalmic use of the amniotic membrane was introduced as late as the 1940s by deRoth who used it in symblepharon (adhesion between eyelid and eyeball) reconstruction.4 However, even though these trials showed the success of the treatment, it was not until the 1990s, when storage methods were found, that the use of amniotic membrane came to be seen as a real possibility.
Amniotic Membrane Structure
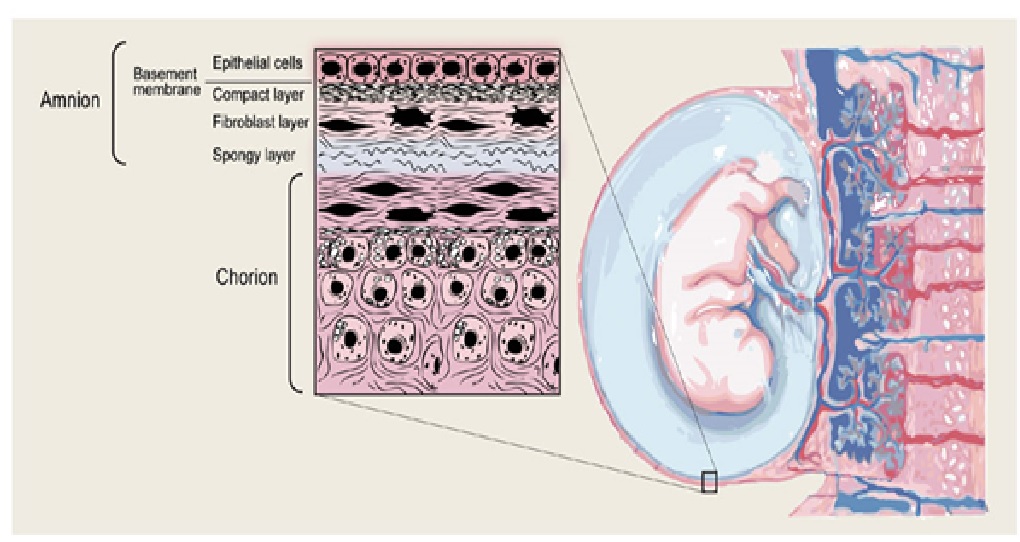
The membranes surrounding the fetus in the uterine cavity are the chorion and amnion. Even though these layers lie close to each other and are very thin, it would be incorrect to understand them as a biologically single layer. The functioning can be accurately understood only by considering them as a bilayer. The chorion is the outer vascular layer that faces the uterine cavity. Inside this lies the amnion, which is an avascular protective layer lining the amniotic fluid.5 The consensus states that the chorion plays a role as an immunological buffer. It stabilizes and prevents the breakdown of the amnion and keeps the fetus is an immune privileged site. The anion is the dominant layer that dictates the mechanical behaviour of the membrane and forms a protective barrier. The amniotic membrane is 0.02-0.05mm thick.1 It is made of a layer of ectodermal columnar cells that are attached to a mesenchymal basement layer with large amounts of collagen.6 On studying the amniotic membrane, it is evident that the ultrastructure has specialized to perform these functions of protection, secretion and intra and trans cellular transport.7
[
Reference5: Bryant-Greenwood GD.
There are grossly three layers- epithelium, stroma and basement membrane. This can be divided into five distinct layers: cuboidal epithelium layer; basement membrane; compact layer; fibroblast layer; and intermediate (spongy) layer.8 The innermost layer has a layer of cuboidal epithelial cells with microvilli. This is attached firmly to a basement membrane made of proteoglycans, mainly heparan sulphate and molecules maintaining its integrity like actin, α-actinin, spectrin, ezrin, several cytokeratins, vimentin, desmoplakin and laminin.9 This is attached further to a collagen layer containing collagen type I, II and V.10 This collagen provides tractional resistance. This layer is followed by a mesenchymal layer composed of fibroblasts. The outermost layer is a collagen III rich spongy layer made of an acellular matrix adjacent to the chorionic leavae.11 Collagen III is responsible for the elasticity of the membrane.
The amniotic membrane is, therefore, not just an avascular membrane. It is responsible for a basic mechanical barrier function. Apart from providing protection, it has a plethora of dynamic functions like transport of water and soluble metabolites, production of vasoactive peptides, growth factors and cytokines.12
Procurement and Storage:
During the process of labour, the membranes rupture and the baby is born. After this, the membranes are extruded along with the placenta. This is the source of amniotic membrane. The extracted membranes are subjected to blunt dissection to separate the amnion from the other layers. Preferably, deliveries by Caesarean section are chosen for this purpose. Vaginal deliveries stand the risk of contamination by the vaginal flora and may subsequently contaminate the procured amnion.
Willing mothers are tested for serology for HIV, hepatitis B and C and syphilis. Some protocols suggest that this HIV testing be repeated thrice so as to rule out the possibility of missing the disease if in window period. The amniotic layer is then separated, washed, sterilized and stored. There are many different protocols for the initial testing and further processing and storage. One method followed was suggested by Kim et al.13 The placenta is first extracted and washed. It is treated by antibiotics in balanced salt solution- 50 mg/ml penicillin, 50 µg/ml streptomycin, 100 mg/ml of neomycin as well as 2.5 mg/ml of amphotericin B. Sterilization is done by peracetic acid and glycerol. Supercritical carbon dioxide may also be used to sterilize the membrane, with the added advantage of preserving all the features of the membrane.14 This membrane is pre cut into required sizes and plated onto nitrocellulose paper with epithelial side up.
The amniotic membrane further can be used fresh or dried and used at a later date. Stored amniotic membrane may be cryopreserved or be stored as a dried, de-epithelialized membrane. The membrane can also be freeze dried or air dried. Cryopreserved amniotic membrane is stored with Dulbecco modified Eagle’s medium or glycerol or cryopreserved at -80oC.15 This retains the moisture and provides antibacterial properties. However, the limited availability of this refrigerator may limit the usage outside big institutions. Before usage, it is essential to thaw this and allow it to come to room temperature. Membrane that is freeze dried with EDTA, vacuum packed and sterilized with gamma-irradiation at 25kGy can be used like the cryopreserved membrane. These are generally dehydrated for storage and can be kept at room temperature for about 5 years. It needs to be rehydrated before it can be used. Lyophilized amniotic membrane is further impermeable to many bacterial strains.16
Fresh and dried-stored amniotic membrane are shown to have similar outcomes in usage.17 A fresh membrane needs to be used immediately which makes the scheduling of surgery difficult. In case of freeze dried membrane, up to 30 units of stored amniotic membrane can be procured from a single placenta, however storage at -70oC limits the widespread use and availability.18 However, usage being at a later date allows the completion of the serology testing, making the procedure safer.
Prokera is a cryopreserved amniotic membrane that is attached to a dual polycarbonate ring and is placed in the eye similar to a contact lens, eliminating the need for sutures or glue.19Â Freeze dried forms commercially available are Ambio2, Ambio5, and AmbioDisk are available in different sizes, are placed on the surgical site while dry, and are activated with sterile saline.20
[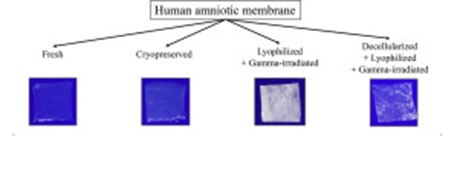
Reference21: Mathilde Fenelon at al
[
Reference22: https://www.biotissue.com/prokera/
Characteristics:
Amniotic membrane has a wide array of properties that make it a very attractive material to be put to use. Foremost among these are that it is avascular, anti-angiogenic and anti-scarring. It promotes healing, epithelialization and produces growth factors. Additionally, it is transparent and non irritant. These provide an added advantage in ocular use.
Firstly, it is a completely natural membrane that poses to ethical issue in usage. Being natural, avascular and devoid of HLA and other antigens, it does not carry a threat of rejection, reaction or non compatibility. It forms a natural barricade and protects the underlying structure from infection and mechanical trauma,23 much like the natural primary role it plays to the fetus. Anti microbial activity is mainly provided by the beta-defensins. Also, low molecular mass elastase inhibitor and elafin are present along with bactricidin, beta-lysin, lysozyme and transferrin,24 which contribute further.
Next, the amniotic membrane shows the presence of fibronectin, elastin, nidogen, collagen, elastin and hyaluronic acid.25 These form a scaffold for epithelialization and proliferation, thereby promoting wound healing. The amniotic basement membrane is made of collagen26 which meticulously resembles the conjunctival and corneal basement membrane. It promotes epithelial cell migration, adhesion and differentiation. It also slows down apoptotic cell death.
The membrane has factors that make it anti-inflammatory and anti-angiogenic. These properties are extremely important in wound healing. The presence of growth factors promotes healing without the formation of a scar or contracture. The anti-angiogenic markers identified are thrombospondin-1, endostatin and tissue inhibitors of metalloproteases (TIMP-1, 2, 3, 4).27 There is inhibition of transforming growth factor (TGFβ-1, 2 and 3). This reduces scar formation. The anti-inflammatory effect of amniotic membrane is due to inhibition of expression of pro-inflammatory cytokines from damaged ocular surface such as interleukin (IL) 1a, IL-2, IL-8, TNF-β, IFN-alpha, FGF and PDGF. This action is done by IL-10 and IL-1RA which are expressed by the amniotic membrane cells.28 The growth factors that have been identified are EGF, TGF and Nerve growth factor (NGF). In addition to this chemical mediated anti inflammatory action, it has also been shown that the membrane mechanically promotes apoptosis of any inflammatory cells that come in contact with it. 29
Recently, amniotic membrane has shown presence of stem cell markers such as OCT-4, Sox-2, FGF-4, Rex-1, CFC1, Nanog, DPPA3, PROM1, PAX6, SSEA-3, SSEA-4,30 Tra 1-60, Tra 1-81 and GCTM2 b. They have the property of pluripotency, clonigenicity and self renewal. This opens a whole new dimension of uses for the membrane. In current use, the presence of pluripotent cells in the membrane make the possibility of phenotypically correct tissue proliferation in the healing wound. It promotes phenotypic transdifferentiation of the host tissue and allows accurate healing.
Surgical Methods:
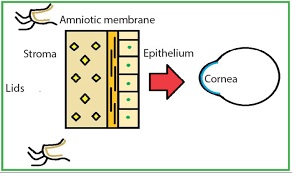
The membrane that is procured on the nitrocellulose paper is placed with the epithelial side up. The orientation of the membrane in the surgery is based on the indication.
The stromal side is identified by the presence of vitreous like strands that can be pulled using forceps.
AM scan be stained with dyes like indocyanine green, rose bengal, trypan blue and lissamine green B. The dyes stain both the epithelial and stromal surfaces. These are important to identify the edges and wrinkles in the graft. Intraoperative staining with lissamine green B may assist surgeons in the proper handling of AM. Fluorescein staining has no role.31
[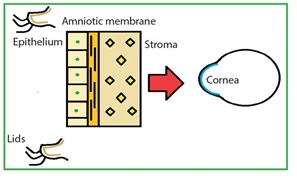
Reference32: Safa Elhassan
This technique is performed in conditions that do not have significant stromal thinning eg. Non healing corneal ulcer, acute ocular burns or chemical injuries and persistent corneal epithelial defects.
[
Reference33: Sangwan VS
The membrane may be sutured to the cornea using 10-0 nylon and to sclera or conjunctiva using 9-0 or 10-0 vicryl. The sutures are anchored to the episclera. Unlike other ocular sutures, the knots of these sutures are left unburied. This is to allow revision and removal of the suture as and when required. Circumferential sutures parallel to limbus or purse string sutures are commonly employed. Each suture is placed parallel to the border of the membrane.
It is important to note that in more extensive diseases like burns or pemphigoid, the amniotic membrane plays an additional role in preventing the formation of adhesions. For this, the membrane needs to placed not just over the defect, but also covering the fornix. Surgeons prefer a large membrane that extends from lid margin to margin covering both the fornices. These large grafts are sutured parallel to the limbus as well as in the fornix.
An additional tarsorrhapy may be performed to prevent early extrusion of the membrane and friction associated damage by the lid movement.
The advent of fibrin glue has reduced the need for sutures. The use of fibrin glue in ophthalmological surface reconstruction is currently done as an off-label use. The use of fibrin glue reduces the surgical time and reduces suture related complications and irritation to the patient, ensuring a faster recovery. There is also a lower rate of membrane shrinkage. The only drawbacks to the usage is the cost of the glue. The combined cost of amniotic membrane and glue makes it economically unviable for widespread use.
Fields of Usage:
Ocular surface reconstruction: 33
Examples from literature:
[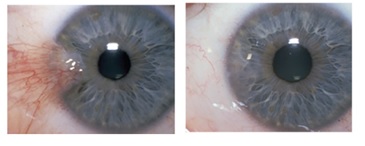
Reference34: Thomas John.
[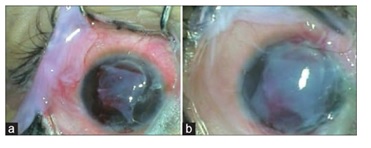
Reference35: Arushi Garg et al
[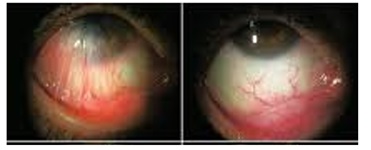
Reference36: Kheirkhah, Ahmad et al
[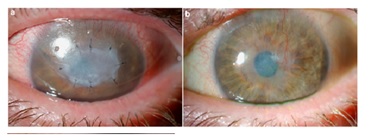
Reference37: Rahman, Irsan & Said, Dalia & Maharajan, Senthil & Dua, Harminder.
[
Reference 38: Guillermo Amescua et al
Pre and post op photos of amniotic membrane procedures performed:
[
[
Limitations:
While there are no limitations of this procedure that warrant an absolute contraindication, the risk-benefit ratio is to be studied and each case individualized to decide on this mode of management.
Amniotic membrane is expensive and is often a surgery that needs to be revised or repeated. The economic impact of this surgery is a factor that needs to be considered before it is made into a standard practice especially among the low socio-economic strata.
Hematoma formation or formation of fluid filled vesicles under the membrane could hamper the healing.
Another fairly common complication that surgeons come across is suture cut through and premature extrusion of the membrane. This may happen intra-operatively as well as the membrane is delicate and difficult to handle. Using toothed forceps and blunt needles are the biggest offenders. Post operatively, the membrane begins to dissolve and may get pulled out of the eye before the necessary healing period.
Calcification of the membrane or residual opaque remnants of the membrane negates the aim of providing a clear optical axis. Luckily, this is quite rare and may be prevented by stringent surgical technique.
A postulated complication that is theoretically very common is the spread of communicable body fluid related diseases like HIV or hepatitis, similar to blood transfusion. However, the infection control measures and serological testing carried out are strict and thorough. Thus practically, this risk is not very high and amniotic membrane grafting is safe in terms of spread of diseases.
If done properly, there is no reason yet to prove why amniotic membrane could be inferior to any other already existing protocol for ocular surface reconstruction and other currently used grafts. However, unequivocal superiority over the same is yet to be established.
Conclusion:
As is clear from the above, amniotic membrane currently has a wide array of uses. This still has scope to be expanded. Research is going in the direction of using the stem cells and scaffolding power of the amniotic membrane in the ex vivo proliferation of epithelial cells and limbal stem cells. This is helpful in the regeneration of a clear cornea.
The magic that the membrane plays in surgical fields is yet to be unfolded to its full capacity. The use of the membrane has shown advantages and good results in would healing and prevents scar formation. Keeping in mind that it is difficult to predict the outcome and difficult to define the procedure as a success or failure, amniotic membrane is still an important tool in treatment unless a similar synthetic membrane is devised with a safety and cure rate profile at par with amniotic membrane. It is evident that the amniotic membrane is here to stay and will find application in a lot more areas as research continues in this field.
References:
1) Malhotra, Chintan, and Arun K Jain. “Human amniotic membrane transplantation: Different modalities of its use in ophthalmology.†World journal of transplantation (2014): 111-21. doi:10.5500/wjt.v4.i2.111
2) Titiyal JS, Falera RC, Kaur M, Sharma V, Sharma N. Prevalence and risk factors of dry eye disease in North India: Ocular surface disease index-based cross-sectional hospital study. Indian J Ophthalmol2018;66:207-11
3) Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910;15:307.
4) deRoth A. Plastic repair of conjunctival defects with fetal membrane. Arch Ophthalmol. 1940;23:522–525
5) Bryant-Greenwood GD. The extracellular matrix of the human fetal membranes:
structure and function. Placenta. 1998 Jan;19(1):1-11
6) Pollard SM, Aye NN, Simmonds EM(1976) Scanning electron microscopic appearance of normal human amnion and umbilical cord at term. Br J ObstetGynaecol 83:470–477
7) van Herendael BJ, Oberti C, BrosensI(1978) Microanatomy of the human amniotic membrane: a light microscopic, transmission and scanning microscopic study. Am J ObstetGynecol 131:872–880
8) Jiang A, Li C, Gao Y, Zhang M, Hu J, Kuang W, et al. In vivo and in vitro inhibitory effect of amniotic extraction on neovascularization. Cornea. 2006; 25:S36–S40
9) Marco Rainer Kesting, Klaus-Dietrich Wolff, Bettina Hohlweg-Majert, Lars Steinstraesser, The Role of Allogenic Amniotic Membrane in Burn Treatment, Journal of Burn Care & Research, Volume 29
10) T. W. Sadler, Langman's Medical Embryology, Slock, London, UK, 8th edition, 2000.
11) Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007 Nov;105(3):215-28
12) Cunningham FG, MacDonald PC, Leveno KJ (2001) Williams obstetrics, 21st edn. McGrawHill, New York
13) Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea 1995;14:473-84
14) J.L. Wehmeyer, S. Natesan, R.J. Christy. Development of a sterile amniotic membrane tissue graft using supercritical carbon dioxide. Tissue Eng C Methods, 21 (7) (2015), pp. 649-659
15) Burgos H, Sergeant RJ. Lyophilised amniotic membranes used in reconstruction of the ear. J R Soc Med 1983;76:433.
16) Nakamura T, Yoshitani M, Rigby H, Fullwood NJ, Ito W, Inatomi T, et al . Sterilized, freeze-dried amniotic membrane: A useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci 2004;45:93-9.
17) Adds PJ, Hunt CJ, Dart JK. Amniotic membrane grafts, "fresh" or frozen? A clinical and in vitro comparison. Br J Ophthalmol2001;85:905-7.
18) Kruse FE, Joussen AM, Rohrschneider K, You L, Sinn B, Baumann J, et al . Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefes Arch Clin Exp Ophthalmol 2000;238
19) Suri K, Kosker M, Raber IM, et al. Sutureless amniotic membrane ProKera for ocular surface disorders: short-term results. Eye Contact Lens. 2013;39(5)
20) Thomasen H, Pauklin M, Noelle B, et al. The effect of long-term storage on the biological and histological properties of cryopreserved amniotic membrane. Curr Eye Res. 2011;36(3)
21) Mathilde Fenelon at al. Comparison of the impact of preservation methods on amniotic membrane properties for tissue engineering applications Materials Science and Engineering. 2019 Nov. 104-10.1016.109903
22) https://www.biotissue.com/prokera/
23) Baum J. Thygeson lecture. Amniotic membrane transplantation: why is it effective? Cornea. 2002;21:339–341
24) GUSDON JP. A bactericidin for Bacillus subtilis in pregnancy.J Immunol. 1962 Apr; 88d1) Grueterich M, Tseng SC. Human limbal progenitor cells expanded on intact amniotic membrane ex-vivo . Arch Ophthalmol2002;120
25) Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of sub-chains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea and conunctiva. Cornea 1999;18
26) Modesti A, Kalebic T, Scarpa S, Togo S, Grotendorst G, Liotta LA, et al . Type V collagen in human amnion is a 12 nm fibrillar component of the pericellular interstitium. Eur J Cell Biol 1984;35
27) Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane.Cornea. 2000; 19(3)
28) Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, Tseng SC. Suppression of Interleukin 1 alpha and Interleukin 1 beta in the human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol2001;85
29) Shimmura S, Shimazaki J, Ohashi Y, Tsubota K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders.Cornea. 2001; 20(4)
30) T. Miki, K. Mitamura, M. A. Ross, D. B. Stolz, and S. C. Strom, “Identification of stem cell marker-positive cells by immunofluorescence in term human amnion,†Journal of Reproductive Immunology, vol. 75, 2007
31) Hu DJ, Basti S, Bryar PJ. Staining characteristics of preserved human amniotic membrane. Cornea 2003;22
32) Safa Elhassan. Understanding amniotic membrane grafts. Ophthalmology, cornea / external eye disease; 2019
33) Sangwan VS, Burman S, Tejwani S, Mahesh SP, Murthy R. Amniotic membrane transplantation: A review of current indications in the management of ophthalmic disorders. Indian J Ophthalmol2007;55
34) Thomas John. Amniotic Membrane Use In Pterygium Surgery. Review of Ophthalmology, 2019
35) Arushi Garg, Kirti Singh, Ankush Mutreja, Keerti Wali, Mainak Bhattacharjee. Amniotic membrane in ophthalmology: A versatile wonder. MAMC Journal of Medical Sciences. 10.4103/2394-7438.166298
36) Kheirkhah, Ahmad et al. Surgical Strategies for Fornix Reconstruction Based on Symblepharon Severity. American Journal of Ophthalmology, Volume 146, 266 - 275.e
37) Rahman, Irsan & Said, Dalia & Maharajan, Senthil & Dua, Harminder. (2009). Amniotic membrane in ophthalmology: Indications and limitations. Eye (London, England). 23. 1954-61. 10.1038/eye.2008.410.
38) Guillermo Amescua, Marwan Atallah, Sotiria Palioura, Victor Perez. Limbal stem cell transplantation: current perspectives. Clinical Ophthalmology. April 2016. 10.2147/opth.s83676
